08 Oct Theory of inert gas dehydrogenation
Theory of inert gas dehydrogenation
What is the theory of inert gas dehydrogenation? The solubility of hydrogen in aluminum is determined by the partial pressure of hydrogen in the gas phase in contact with the aluminum melt. When the hydrogen partial pressure in the equilibrium state corresponding to the hydrogen content in the aluminum melt is greater than the hydrogen partial pressure in the actual gas in contact with the aluminum melt, the hydrogen in the aluminum melt will diffuse into the gas phase to achieve hydrogen removal. This is the principle of hydrogen removal by bubble flotation. According to this, pure inert gas is introduced into the melt, because the partial pressure of hydrogen in the inert bubble is P’H2=0, and the hydrogen partial pressure in the equilibrium state corresponding to the hydrogen content in the melt is PH2>0, the hydrogen in the melt will Diffusion into the bubbles, compounded into hydrogen molecules and discharged from the melt with the bubbles.
In order to remove as much hydrogen as possible from the aluminum melt with as little inert gas as possible, the following principles should be followed for online inert gas dehydrogenation:
(1) the lower the content of hydrogen and oxygen in the inert gas, the better;
(2) The more bubbles formed by blowing the inert gas in the melt, the finer and the more dispersed the better;
(3) the longer the bubbles stay in the aluminum melt, the better.
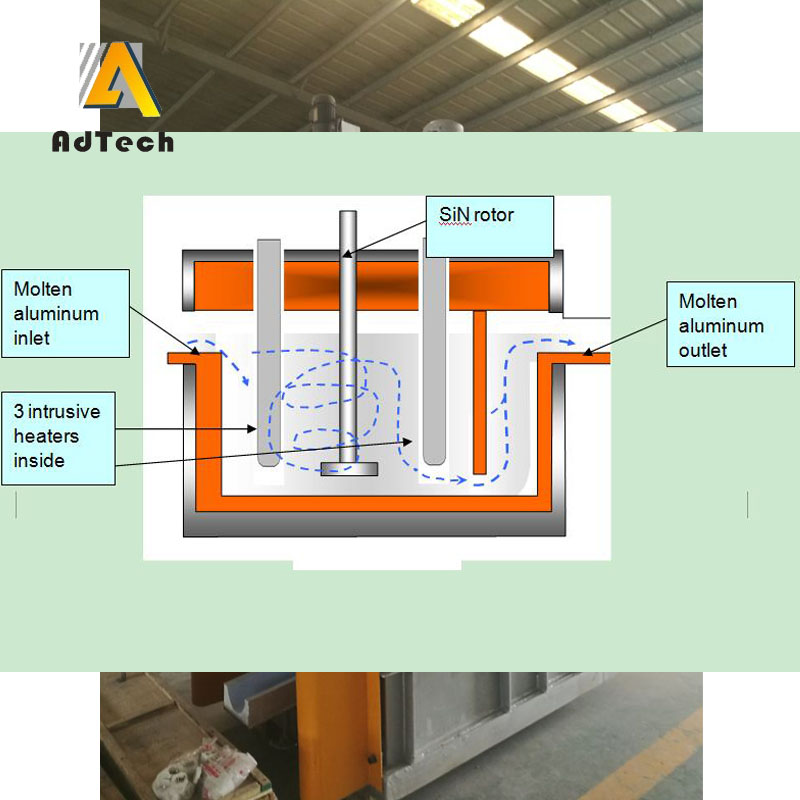
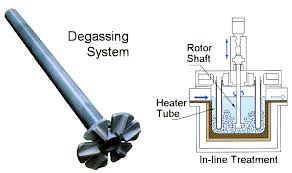
For this reason, the inert gas degassing unit mostly uses high-speed rotating nozzles, and the fluid ejected from the nozzle blades produces a strong turbulent flow. The bubbles ejected from the high-speed rotating nozzle are rapidly sprayed by the melt sprayed by the nozzle blades and the surrounding relatively slow melt near the nozzle.
Captured, the bubble is subjected to the turbulent shear stress generated by the two layers of fluid, and when it is greater than the bearing capacity of the bubble, it is cut off and split into two bubbles. The split bubbles are then captured by other tangential liquid layers and can continue to split into smaller bubbles until the shear stress between the two layers of fluids is not enough to break the bubbles or the bubbles are out of the range of this shear stress.
Thereby, finer and more uniform bubbles can be generated than single-tube or multi-tube, which can effectively increase the specific surface area, and also effectively increase the mass transfer and diffusion capacity, and at the same time prolong the gas. The contact time of the liquid interface, when the intake flow of the purification gas is constant, makes the concentration of hydrogen in the purification bubbles escaping from the aluminum melt larger, and the purification gas is effectively used so that an excellent purification effect can be obtained.














No Comments